Atoms make up ordinary matter. In 1897 J. J. Thomson discovered the electron, a negatively charged particle more than two thousand times lighter than a hydrogen atom. In 1906 Thomson suggested that each atom contained a number of electrons roughly equal to its atomic number. Since atoms are neutral, the charge of these electrons must be balanced by some kind of positive charge. Thomson proposed a 'plum pudding' model, with positive and negative charge filling a sphere ~10-10 meter across. This plum pudding model was generally accepted. Even Thomson's student Rutherford, who would later prove the model incorrect, believed in it at the time.
But in 1911 Ernest Rutherford proposed that each atom has a massive nucleus containing all of its positive charge, and that the much lighter electrons are outside this nucleus. The nucleus has a radius about ~ 10-14 - 10-15 m, ten to one hundred thousand times smaller than the radius of the atom. Rutherford arrived at this model by doing experiments. He scattered alpha particles off fixed targets and observed some of them scattering through very large angles. Scattering at large angles occurs when the alpha particles come close to a nucleus. The reason that most alpha particles are not scattered at all is that they are passing through the relatively large 'gaps' between nuclei.
Is the energy of the ground state (i.e., the lowest energy state). Here, is a positive integer that must exceed the quantum number, otherwise there would be no terms in the series ( 4.126 ). Expression ( 4.132 ) is known as the Bohr formula, because it was derived heuristically by Niels Bohr in 1913 10. Mechanical nature of the hydrogen atom helps us understand how these lines arise. Series of lines in the hydrogen spectrum, named after the scien-tists who observed and characterized them, can be related to the energies associated with transsitions from the various energy lev-els of the hydrogen atom. The relation, simple enough as it is.
- For a hydrogen atom of a given energy, the number of allowed states depends on its orbital angular momentum. We can count these states for each value of the principal quantum number, n = 1, 2, 3. However, the total energy depends on the principal quantum number only, which means that we can use Equation 8.2.5 and the number of states counted.
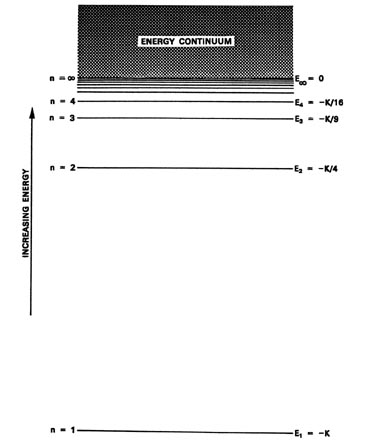
- The fact that hydrogen atoms emit or absorb radiation at a limited number of frequencies implies that these atoms can only absorb radiation with certain energies. This suggests that there are only a limited number of energy levels within the hydrogen atom. These energy levels are countable. The energy levels of the hydrogen atom are quantized.
Links: (animations)
The Rutherford Experiment
Thomson Model of an Atom
Rutherford Model
Rutherford revised Thomson's 'plum pudding' model, proposing that electrons orbit a positively charged nucleus, like planets orbit a star. But orbiting particles continuously accelerate, and accelerating charges produce electromagnetic radiation. According to classical physics the planetary atom cannot exist. Electrons quickly radiate away their energy and spiral into the nucleus.
In 1915 Niels Bohr adapted Rutherford's model by saying that the orbits of the electrons were quantized, meaning that they could exist only at certain distances from the nucleus. Bohr proposed that electrons did not emit electromagnetic radiation when moving in those quantized orbits.
What do our instruments reveal today? Here are 3 examples.
All the examples reveal the probability distribution of atomic electrons, i.e. the probability of finding an electron at certain positions near an atomic nucleus.
The field ion microscope and the STM look at atomic cores that are fixed in the crystal structure of a conductor. By different means they supply barely enough energy to remove the electron from the conductor's surface The electron is most likely removed from positions near the atomic cores. The removed electron is replaced by a small current flowing to the ground. So electrons can bee repeatedly removed from the same atoms and plotting the number of electrons removed or the current flowing versus position maps the electron probability distribution.
| Field Ion microscope | Scanning-Tunneling microscope (STM) |
The quantum microscope works with a beam of atoms. Electrons are removed from different, identically prepared atoms. A electron optics system images their removal position onto a detector. Each atom only contributes one lectron. but after a sufficient number of electrons has been detected, a very detailed probability distribution emerges.,
Quantum mechanics now predicts what measurements can reveal about atoms. The hydrogen atom represents the simplest possible atom, since it consists of only one proton and one electron. The electron is bound, or confined. Its potential energy function U(r) expresses its electrostatic potential energy as a function of its distance r from the proton.
U(r) = -qe2/(4πε0r).

In SI unit 1/(4πε0) = 9*109 Nm2/C2, and qe = 1.6*10-19 C.
The figure on the right shows the shape of U(r) in a plane containing the origin. The potential energy is chosen to be zero at infinity. The electron in the hydrogen atom is confined in the potential well, and its total energy is negative.
Confinement leads to energy quantization.
The allowed energies of the electron in the hydrogen atom are
En = -13.6 eV/n2.
Here n is called the principle quantum number. The values En are the possible value for the total electron energy (kinetic and potential energy) in the hydrogen atom. The average potential energy is -2*13.6 eV/n2 and the average kinetic energy is +13.6 eV/n2. The electron has four degrees of freedom, the three spatial degrees of freedom and one internal degree of freedom, called spin. To completely determine its initial wave function, we, in general, have to make four compatible measurements.
Some observables that are compatible with energy measurements and compatible with each other are
- the magnitude of the electron's orbital angular momentum, labeled by the quantum number l,
L = (l(l + 1))1/2ħ, - the projection of the electron's orbital angular momentum along one axis, for example the z-axis, labeled by the quantum number m,
Lz = mħ, - and the projection of the electron's spin along one axis, labeled by by the quantum number ms,
Sz = msħ.
We can know the values of these observables, labeled by n, l, m, and ms, simultaneously.
For the hydrogen atom, the energy levels only depend on the principal quantum number n. The energy levels are degenerate, meaning that the electron in the hydrogen atom can be in different states, with different wave functions, labeled by a different set of quantum numbers, and still have the same energy.
The electron wave functions however are different for every different set of quantum numbers.
- For each principal quantum number n, all smaller positive integers are possible values for the quantum number l, i.e. l = 0, 1, 2, .., n - 1. The quantum number l is always smaller than the quantum number n. Only states with high energy can have large angular momentum.
- The quantum number m can take on all integer values between -l and l.
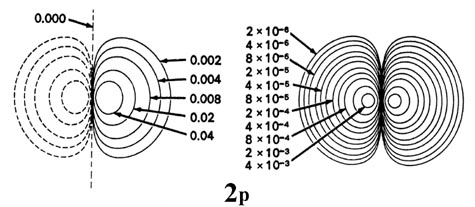
Examples of hydrogen atom probability densities.
As n increases, |
|
Note: Energy eigenfuctions characterize stationary state. We cannot track the electron and know its energy at the same time. If we know its energy, we can only predict probabilities for where we might find it if we tried to measure its position. If we determine the position of the electron, we lose the energy information.
| l = 1 | s |
| l = 2 | p |
| l = 3 | d |
| l = 4 | f |
| l = 4 | g |
Often texts use a different notation to refer to the energy levels of the hydrogen atom. Letters of the alphabet are associated with various values of l.
| Spectroscopic notation | Quantum number n of the state | Quantum number l of the state | Possible values of the quantum number m |
|---|---|---|---|
1s | 1 | 0 | 0 |
2s | 2 | 0 | 0 |
2p | 2 | 1 | -1, 0, 1 |
3s | 3 | 0 | 0 |
3p | 3 | 1 | -1, 0, 1 |
3d | 3 | 2 | -2, -1, 0, 1, 2 |
4s | 4 | 0 | 0 |
4p | 4 | 1 | -1, 0, 1 |
4d | 4 | 2 | --2, -1, 0, 1, 2 |
4f | 3 | -3, -2, -1, 0, 1, 2, 3 |
The hydrogen line spectrum
When an electron changes from one energy level to another, the energy of the atom must change as well. It requires energy to promote an electron from a lower energy level to a higher one. This energy can be supplied by a photon whose energy E is given in terms of its frequency E = hf or wavelength E = hc/λ.
Since the energy levels are quantized, only certain photon wavelengths can be absorbed. If a photon is absorbed, an electron will be promoted to a higher energy level and will then fall back down into the lowest energy state (ground state) in a cascade of transitions. Each time the energy level of the electron changes, a photon will be emitted and the energy (wavelength) of the photon will be characteristic of the energy difference between the initial and final energy levels of the atom in the transition. The energy of the emitted photon is just the energy difference between the initial (ni) and the final (nf) state.

The set of spectral lines for a given final state nf are generally close together. In the hydrogen atom they are given special names. The lines for which nf = 1 are called the Lyman series. These transitions frequencies correspond to spectral lines in the ultraviolet region of the electromagnetic spectrum. The lines for which nf = 2 are called the Balmer series and many of these spectral lines are visible. The spectrum of hydrogen is particularly important in astronomy because most of the Universe is made of hydrogen. The Balmer series, which is the only hydrogen series with lines in the visible region of the electromagnetic spectrum, is shown in the right in more detail.
The Balmer lines are designated by H with a Greek subscript in order of decreasing wavelength. Thus the longest wavelength Balmer transition is designated H with a subscript alpha, the second longest H with a subscript beta, and so on.
Problem:
What is the wavelength of the least energetic line in the Balmer series?

Solution:
- Reasoning:
The transition from ni = 3 to nf = 2 is the lowest energy, longest wavelength transition in the Balmer series. - Details of the calculation:
∆E = -13.6 eV(1/9 - 1/4) = 1.89 eV = 3*10-19 J. λ = hc/∆E = 658 nm.
Problem:
What is the shortest wavelength in the Balmer series?
Solution:
- Reasoning:
The transition from ni = ∞ to nf = 2 is the highest energy, shortest wavelength transition in the Balmer series. - Details of the calculation:
∆E = -13.6 eV(1/∞ - 1/4) = 13.6/ 4 eV = 3.4 eV = 5.44*10-19 J. λ = hc/∆E = 365 nm.
Problem:
Do the Balmer and Lyman series overlap? To answer this, calculate the shortest-wavelength Balmer line and the longest-wavelength Lyman line.
Solution:
- Reasoning:
From the previous problem we know that the shortest-wavelength Balmer line has λ = 365 nm.
The transition from ni = 2 to nf = 1 is the lowest energy, longest wavelength transition in the Lyman series. - Details of the calculation:
∆E = -13.6 eV(1/2 - 1) = 13.6/ 2 eV = 6.8 eV. λ = hc/∆E = 182 nm.
The two series do not overlap.
Module 11, Question 1
- Hydrogen gas can only absorb EM radiation that has an energy corresponding to a transition in the atom, just as it can only emit these discrete energies. When a spectrum is taken of the solar corona, in which a broad range of EM wavelengths are passed through very hot hydrogen gas, the absorption spectrum shows all the features of the emission spectrum. But when such EM radiation passes through room-temperature hydrogen gas, only the Lyman series is absorbed. Explain the difference.
Discuss this with your fellow students in the discussion forum!
Hydrogenic atoms
Atoms with all but one electron removed are called hydrogenic atoms.
- If the charge of the nucleus is Z times the proton charge, then U(r) = -Zqe2/(4πε0r).
- The energy levels of such atoms are obtained by simply scaling the the solutions for the hydrogen atom.
The energy levels scale with Z2, i.e. En = -Z2*13.6 eV/n2. It takes more energy to remove an electron from the nucleus, because the attractive force that must be overcome is stronger. - The average size of the wave functions scales as 1/Z, i.e. the electron, on average, stays closer to the nucleus, because the attraction is stronger.
Problem:
Atoms can be ionized by thermal collisions, such as at the high temperatures found in the solar corona. One such ion is C+5 , a carbon atom with only a single electron.
(a) By what factor are the energies of its hydrogen-like levels greater than those of hydrogen?
(b) What is the wavelength of the first line in this ion's Paschen series?
(c) What type of EM radiation is this?
Solution:
- Reasoning:
The energy levels of hydrogenic atoms scale with Z2, the wave functions scales as 1/Z. - Details of the calculation:
(a) The charge of the carbon nucleus is 6 times the proton charge. All energy level increase by a factor of Z2 = 36.
(b) λ = hc/∆E, all wavelengths of the Paschen series of carbon decrease by a factor of 36 compare to the corresponding wavelengths of hydrogen.
For hydrogen the first line of the Paschen series has ∆E = -13.6 eV(1/16 - 1/9) = 0.66 eV, λ = hc/∆E = 1876 nm.
For carbon the first line of the Paschen series has λ = hc/∆E = 1875/36 nm = 52.1 nm.
(c) This is far UV radiation.
The Bohr Atom
In 1913 Bohr's model of the atom revolutionized atomic physics. The Bohr model consists of four principles:
- Electrons assume only certain orbits around the nucleus. These orbits are stable and called 'stationary' orbits. Electrons in these orbits do not radiate their energy away.
- Each orbit is associated with a definite value of the energy and the angular momentum. Bohr assumed that the angular momentum could only take on values that were integer multiples of ħ.
Angular momentum = mr2ω = mrv = nħ, n = 1, 2, 3, .. .
A classical electron with a definite angular momentum in an orbit about a proton also has a definite energy E .
If angular momentum = mrv = nħ, then En = -me4/(2ħ2n2) = -13.6 eV/n2.
The orbit closest to the nucleus has an energy E1, the next closest E2 and so on.
A definite angular momentum also implies a definite orbital radius.
If angular momentum = mrv = nħ, then rn = n2ħ2/(me2) = n2a0 = n2 * (52.92 pm).
a0 is called the Bohr radius. - A photon is emitted when an electron jumps from a higher energy orbit to a lower energy orbit and absorbed when it jumps from a lower energy orbit to higher energy orbit. The photon energy is equal to the energy difference ∆E = hf = Ehigh - Elow.
With these conditions Bohr was able to explain the stability of atoms, as well as the emission spectrum of hydrogen. According to Bohr's model only certain orbits are allowed, which means only certain energies are possible. These energies naturally lead to the explanation of the hydrogen atom spectrum.
Energy Of Hydrogen Atom At Ground State
Bohr's model was so successful that he immediately received world-wide fame. Unfortunately, Bohr's model worked only for hydrogen and hydrogenic atoms, such as any atom with all but one electron removed.
The Bohr model is easy to picture, but we now know that it is wrong. Any planetary model of the atom, so often seen in pictures and so easy to picture, is wrong. Energy and position are incompatible observables. We cannot track an electron with a known energy inside an atom.
Energy Of Hydrogen Atom In First Excited State
